Overwhelming feelings of sadness, despair, worthlessness, and loneliness that affect sleep, diet, social relations and daily functioning are common in those with depression.
Transcranial Magnetic Stimulation (TMS) is FDA-approved and covered by many insurances for Multi-Medication Resistant Major Depressive Disorder in adults. TMS therapy has shown a clinically significant response in up to 80% of individuals who did not experience beneficial results from anti-depressants. About one-third of these individuals experience full remission.
MeRT is an individualized application of TMS that tailors treatment to meet the unique needs of your brain.


ASSESS
Begin with a call to our patient coordinator who will collect your depression history. Then meet with our clinician who will assess your candidacy for TMS. We will next work with you on applying for TMS insurance coverage as well as collect pre-treatment data.
TREAT
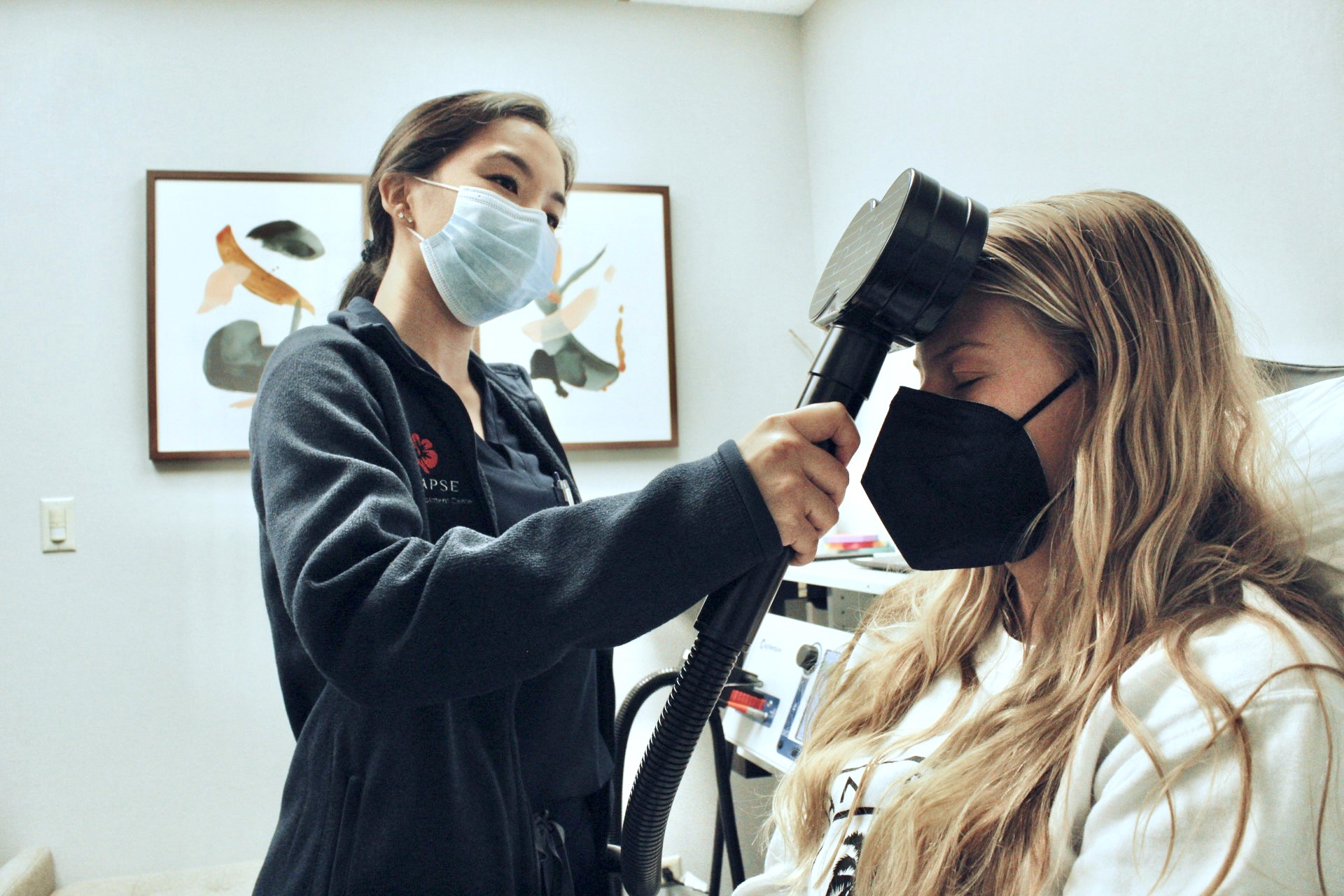
For each session, an FDA-approved, 19-minute Magventure TMS treatment protocol is delivered in our office. During your treatment, you will sit in a comfortable chair. A neuro-technician will place a magnetic coil on your head and monitor you during treatment. This device produces a low-frequency magnetic field to induce mild electrical signals in the targeted areas of the brain. We recommend patients to plan for 30 sessions of TMS treatment over the course of 6 weeks (Monday-Friday) for best results.
Progress Monitor & Evaluate
For each session, an FDA-approved, 19-minute TMS treatment protocol is delivered in our office. During your treatment, you will sit in a comfortable chair while a highly trained neuro-technician places a magnetic coil on your head. This device produces a low-frequency magnetic field to induce mild electrical signals in the targeted areas of the brain. We recommend patients to plan for 30 sessions of TMS treatment over the course of 6 weeks (Monday-Friday) for best results.
ASSESS
Begin with a consultation with our clinician and have your qEEG taken, analyzed, and interpreted. These steps will help determine if MeRT is a good fit for you.
TREAT
A personalized treatment protocol is developed based on your qEEG. Per your protocol, gentle magnetic waves are applied to targeted areas of your brain in our office by one of our technicians. Each treatment period consists of 10 daily MeRT treatments over the course of 2 weeks (Monday-Friday).
Progress Monitor & Evaluate
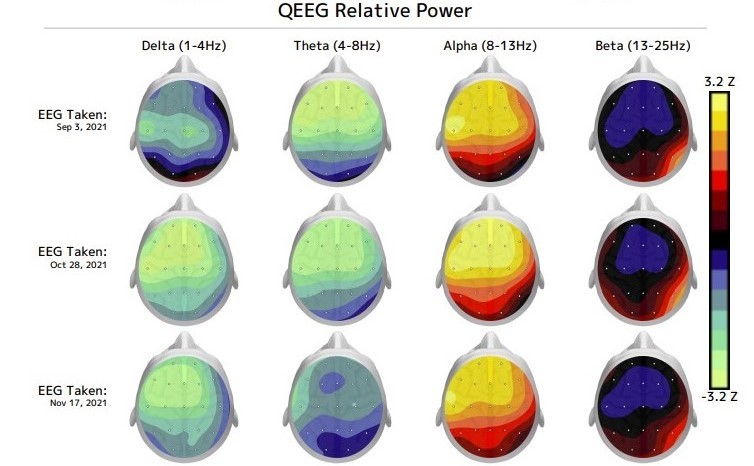
Near the end of every 2-week treatment period, a follow up qEEG, clinical scales, and a clinician consultation is done to evaluate your progress and guide additional treatment.
THE RESEARCH
- Depression
- The Relationship Between Brain Oscillatory Activity and Therapeutic Effectiveness of Transcranial Magnetic Stimulation in the Treatment of Major Depressive Disorder
- Brain Activity and Clinical Outcomes in Adults with Depression Treated With Synchronized Transcranial Magnetic Stimulation: An Exploratory Study
- Efficacy and Safety of Low Field of Synchronized Transcranial Magnetic Stimulation (sTMS) for Treatment of Major Depression
- A Pilot Study of the use of EEG-based Synchronized Transcranial Magnetic Stimulation (sTMS) for treatment of Major Depression
- Experimental Depression Treatment is Nearly 80% Effective in Controlled Study
- Predictors of Response to Synchronized Transcranial Magnetic Stimulation for Major Depressive Disorder
- A Systematic Review and Meta-Analysis on the Efficacy and Acceptability of Bilateral Repetitive Transcranial Magnetic Stimulation (rTMS) for Treatment Major Depression
- Recovering from Depression with Repetitive Transcranial Magnetic Stimulation (rTMS): A Systematic Review and Meta-Analysis of Preclinical Studies
- Response Remission and Drop-Out Rates Following High-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) for Treating Major Depression: A Systematic Review and Meta-Analysis of Randomized, Double-Blink and Sham-Control Trials
- Synchronized Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder and Comorbid Major Depression
TESTIMONIALS
MeRT FAQ
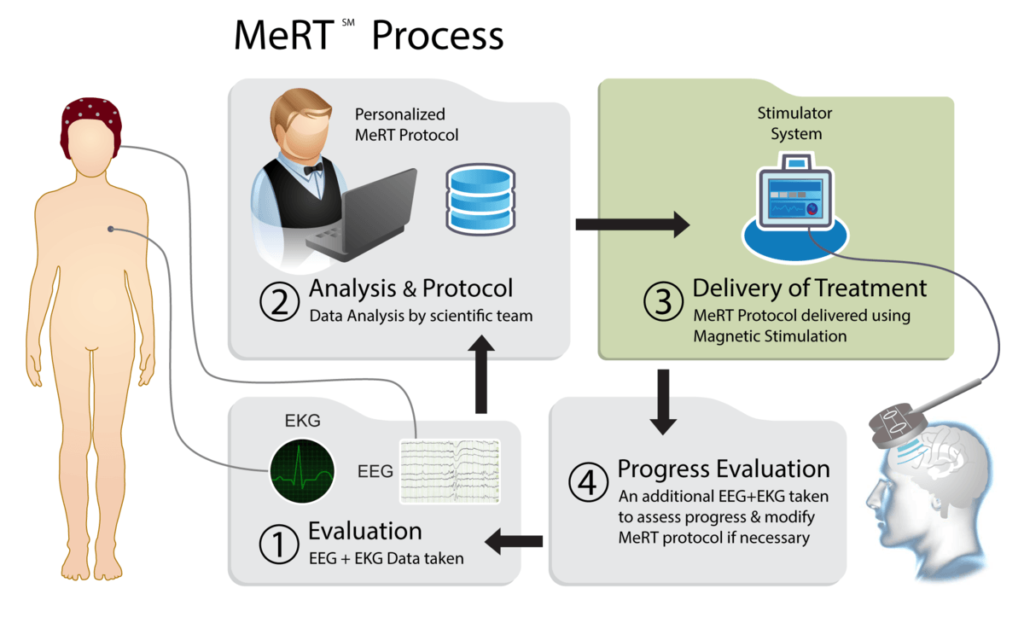
MeRT stands for Magnetic qEEG-guided Resonance Therapy. MeRT is a treatment that combines repetitive transcranial magnetic stimulation (rTMS, an FDA cleared therapy), Quantitative Electroencephalogram (qEEG), and Electrocardiogram (ECG/EKG) to deliver treatments tailored for each individual’s unique brain pattern.
The patient’s qEEG and EKG are analyzed to determine the brain’s pattern of function and activity. The resulting information is used to develop a personalized treatment aimed at shifting brain connectivity to an individual’s ideal brainwave frequency, which can lead to significant clinical improvements.
Repetitive Transcranial Stimualtion (rTMS) therapy delivers a standardized treatment approach that uses the same protocol by stimulating the same location of the brain at a fixed frequency for all patients. MeRT delivers an individualized treatment approach that varies in treatment location, treatment frequency, and stimulation intensity based on each patient’s quantitative EEG and EKG data.
MeRT’s intensity of stimulation is lower than rTMS since less intense magnetic stimulation is needed to generate the same level of results due to MeRT’s use of individually tailored treatment location(s) and frequencies. Thus, less side effects are associated with MeRT compared to rTMS.
At Mālama Manaʻo, we require a diagnosis for individuals seeking treatment for Autism Spectrum Disorder prior to starting MeRT. For all other conditions, a diagnosis is not necessary to start MeRT. However, for those seeking insurance coverage for multi-medication resistant depression, a diagnosis is required. We also highly recommend all individuals receive diagnostic assessment and establish care with appropriate providers.
Absolute contraindications for cortical MeRT treatment at Mālama Manaʻo:
- Pacemaker
- Defibrillator
- Vagal Nerve Stimulator
- VP Shunt/ Magnetic intracranial shunts
- Deep Brain Stimulator
- Epidural Cortical stimulator
- Steel shunts/stents
- Cranial metal fragments (i.e. shrapnel, excluding titanium)
- Schizophrenia or history of psychosis (i.e. hallucinations, delusions)
- Cochlear implant
- Aneurysm clips, coils, pipelines flow diversion
- Pregnant or breastfeeding
- Primary brain cancer/metastatic lesions in brain (unless palliative care)
- Magnetic dental implants
- Implanted cardio-verter defibrillators (ICD)
- Ocular implants
- Untreated seizure activty
- *Bipolar Disorder Type I/II
- *Schizophrenia or Schizoaffective Disorder
- *Active Suicidal Ideation
*May be treated at other TMS/MeRT centers, but not at Mālama Manaʻo
Relative contraindications (requiring closer protocol attention, but not disqualifying someone from receiving cortical MeRT℠ treatment:):
- History of Seizure or seizure disorder
- Titanium shunts/stents
- Spinal Cord Stimulator
- Hearing aids
- Ferrous cortical implants
- Magnetic ink tattoo
- Bipolar Disorder Type I/II
- Baha Implant
We currently only provide qEEG, consultation, and treatment services related to MeRT at Mālama Manaʻo. Diagnostic assessment and any other services are not available at this time.
We can connect you with our parent company Manakai O Mālama Integrative Healthcare Group and Rehabilitation Center’s behavior health team who offers diagnostic assessment and therapy for adults.
Common benefits of MeRT include reduction of symptoms and behaviors interfering with daily functioning and the following:
- Better sleep
- More relaxed; less anxiety
- Mood improvement and increased emotional stability
- Increased ability to adapt to change
- Improved self-confidence, self-esteem, and sociability
- More “presence” with Autistic patients (interaction and awareness with the world around them)
- Better concentration and focus
- Improved memory
- Greater ability to cope with stress
- Increased motivation
- Decreased cravings for drugs and alcohol
Results are individual in nature, depending on the initial presenting symptoms, and are not guaranteed.
Side effects are few, generally mild, and short-lived. The most commonly reported side effect is a mild tension headache, which typically responds well to over-the-counter painkillers. Other possible side effects are hyperactivity, fatigue, increased agitation, or euphoria. If side effects occur during treatment, inform your technician so that treatment may be adjusted accordingly.
With brain stimulation there is also a risk of seizures; however the risk is minimal (1: 100,000) with MeRT therapy. At Mālama Manaʻo, MeRT treatment protocols take into account past or present seizure activity to reduce the risk of seizure caused by treatment.
MeRT is painless and non-invasive. MeRT stimulation is accompanied by a clicking noise and often feels like light to firm tapping in the stimulation area. If any discomfort is reported by a patient, our technicians are trained to adjust treatment intensity accordingly.
Every individual’s response to treatment is different. We require every new patient to undergo a 2 week assessment period. Generally, most people experience some sort of benefit within the first 2-3 weeks of treatment.
We recommend that you continue taking your prescribed medications while receiving MeRT and do not add, stop, or change any medications while receiving MeRT to ensure accurate progress monitoring. Please inform our clinician of all medications you are taking prior to starting treatment and let your technician know if any changes in medication occur during treatment.
Patients may choose to opt for additional treatment in the future if they wish to “refresh” benefits or pursue additional benefits. We recommend that patients take a 3 month break before pursing additional MeRT.
After your initial consultation and baseline assessment of a qEEG and qEEG review, treatment is scheduled in 10 treatment session intervals.
Treatment sessions are once per day, five days per week (Monday through Friday), at the same time every day over the course of two weeks. Each treatment session lasts approximately 30-45 minutes.
A follow-up qEEG and qEEG review with the clinician is scheduled near the end of each 10 treatment period to monitor your progress and inform continued care.
It is recommended that individuals complete at least four to six consecutive weeks of MeRT in order to achieve maximum, long-lasting benefits.
We highly recommend that you pursue other modalities of treatment for you or your loved one’s condition in addition to MeRT. Best practice for most health conditions is a multimodal approach as there are various components to treating different conditions (i.e. medical, psychological, educational, environmental, etc.). Many past patients report that MeRT increased their rate and level of benefits in other forms of treatment such as counseling, Applied Behavior Analysis, neurofeedback, etc. which increased their total level of clinical gains(s).
Our office is in the Gold Bond Building at 677 Ala Moana Blvd Honolulu, Hawaii 96813 within the Manakai O Malama office.
The Manakai O Mālama office is on the 9th floor Suite 950. We recommend patients to enter the parking garage from Coral St. and use the Ewa Wing elevators.
You may park in the Gold Bond Building parking lot on Ala Moana Blvd at 677 Ala Moana Blvd. Honolulu, HI 96813. We recommend entering the parking garage from Coral St. and using the Ewa Wing elevators. Parking validation for a discounted rate ($1.75/hour) is available.
Financial FAQ
If insurance coverage is not available, out of pocket costs for baseline assessment TMS nd MeRT services are listed below:
~Baseline MeRT qEEG assessment: $200
~TMS and MeRT Clinician Consultation: $150
~TMS & MeRT Treatment: First 10 sessions $350 each. Additional sessions $250 each.
*For more information about cost of treatment, please call our patient coordinator. We are happy to work with you to figure out payment options, if insurance does not cover treatment for you.
We are established with HMSA, Tricare, Aloha Care, Ohana Health Plan, HMAA, HMA, Medicare, Medicaid, and more.
Repetitive Transcranial Magnetic Stimulation (rTMS), which MeRT employs, is FDA-cleared and covered by most major health insurances to treat those with multi-medication resistant Major Depressive Disorder (MDD) in adults 18 years and older. To qualify and receive insurance coverage for rTMS under multi-treatment resistant Major Depressive Disorder (MDD), the following guidelines must be met (more specific guidelines may vary by insurance provider):
1) A confirmed diagnosis of Major Depressive Disorder
2) Multiple failed trials of pharmacological intervention and a failed trial of psychotherapy
3) Order from a psychiatrist
*If you are missing any of the above guidelines but believe you have depression and would like TMS/MeRT therapy, our clinician can work with you to complete the above guidelines to qualify for insurance coverage.
Treatment for all other conditions is considered “off-label” and requires payment out of pocket. Insurance reimbursement may be available for qEEGs and physician consultations depending on your health coverage. Past patients were able to use Flexible Spending to pay for TMS/MeRT.
Most insurances cover TMS, some insurances also cover MERT, for treatment of multi-medication resistant Major Depressive Disorder (MDD) in adults under the following guidelines (more specific guidelines may vary by insurance provider):
1) A confirmed diagnosis of severe Major Depressive Disorder
2) 2+ failed trials of pharmacological intervention and 1+ failed trial of psychotherapy
3) Order from a psychiatrist
If you are seeking insurance covered TMS or MeRT for depression, have your psychiatrist or psych APRN submit a TMS referral with clinical notes to Manakai O Malama.
Referrals may be sent to:
Fax: 808-535-5556
Manakai O Malama Integrative Healthcare Group and Rehabilitation Center
*If you are missing any of the above insurance guidelines but believe you have depression and would like TMS/MeRT therapy, our clinician can work with you to complete your plan’s TMS guidelines to qualify for TMS insurance coverage.
For those seeking treatment for other conditions, a referral is not needed.
Please contact our patient coordinator at 808-754-1027 or brainwellness@manakaiomalama.com, if you have any questions.
Thank you for your service!
Tricare covers TMS and MeRT treatment for adults with multi-treatment resistant Major Depressive Disorder. For those with Tricare Prime, please have your primary care provider (PCP) submit referrals for TMS and “Specialty Evaluate and Treat” with a depression diagnosis to Manakai O Malama. Please also have your PCP or mental health physician (must be an MD) sign and submit a TMS Letter of Attestation to Tricare to expedite insurance prior authorization review. Please contact us directly after you request a referral from your PCP, so that we may follow up on your referral. Once Tricare authorizes treatment, we will begin scheduling you for your first appointment.
Referrals may be sent to:
Fax: 808-535-5556
Manakai O Malama Integrative Healthcare Group and Rehabilitation Center
For Tricare Select or Reserve, a referral is not necessary. You may contact our office to begin scheduling your first appointment.
Acceptable forms of payment are cash ($), check, and major credit cards including Visa, MasterCard, American Express, and Discover. There is a $25 processing fee for returned checks. If you have any questions or concerns regarding payment, please contact our patient coordinator. They are available at 808-754-1027 or brainwellness@manakaiomalama.com.